Rules of Thumb—Wastewater Treatment
Updated: Sep 9, 2023
Some Simple Guides
This post provides tips and insights I've come across focused on wastewater treatment topics. A search on Google for “Rule of Thumb” leads to the following definition:
“A broadly accurate guide or principle, based on experience or practice rather than theory.”

Best Wastewater Treatment Option Based on Organic Concentration
Here is one of my favorite, very handy, Rules-Of-Thumb, quoted from the excellent reference provided below:
"Aerobic cultures of microorganisms are particularly suitable for the removal of organic matter in the concentration range between 50 and 4,000 mg/L as biodegradable chemical oxygen demand (COD). At lower concentrations, carbon absorption is often more economical, although biochemical operations are being used for treatment of contaminated groundwaters that contain less than 50 mg/L of COD. Although they must often be followed by aerobic cultures to provide an effluent suitable for discharge, anaerobic cultures are frequently used for high strength wastewaters because they do not require oxygen, give you less excess biomass, and produce methane gas as a usable product. If the COD concentration to be removed is above 50,000 mg/L, however, then evaporation and incineration may be more economical. Anaerobic cultures are also used to treat wastewaters of moderate strength (down to ~1,000 mg/L as COD), and have been proposed for use with dilute wastewaters as well. It should be emphasized that the concentrations given are for soluble organic matter. Suspended or colloidal organic matter is often removed more easily from the main wastewater stream by physical or chemical means, and then treated in a concentrated form. However, mixtures of soluble, colloidal, and suspended organic matter are often treated by biochemical means."
Source: Grady, C.P. Leslie Jr., Glen T Daigger, and Henry C. Lim. "Biological Wastewater Treatment." Second Edition. New York: Marcel Dekker, Inc., 1999.
It should be emphasized that the concentrations given are for soluble organic matter.

Based on the quote above from Grady, Daigger, and Lim, here is my attempt to provide a simple tabulation of the information.

Here's another source of information to guide your decision-making based on the organic concentration of the wastewater to be treated.
Wastewater concentration is not, in itself, a technical barrier to the implementation of anaerobic treatment. In general terms, full-scale experience has shown that anaerobic treatment is most suitable for wastewaters with biodegradable Chemical Oxygen Demand (COD) concentrations in the intermediate-to-high strength range from 2,000 to >20,000 mg COD/L.
Considerations Related to Wastewater Concentration
COD <1,000 to 2,000 mg/L
High-rate anaerobic treatment may be preferred
Residual COD may be relatively high after anaerobic treatment
Aerobic or physical/chemical post-treatment may be required
Economics my favor 100% aerobic treatment
Insufficient methane production for maintenance of reactor temperature
COD >20,000 mg/L
Low-rate anaerobic treatment may be preferred
Effluent quality may be poor unless biosolids are removed
Physical/chemical treatment options could be preferred
Since anaerobic processes leave a relatively high residual of undegraded organics in treated effluents, anaerobic treatment alone rarely results in BOD (Biochemical Oxygen Demand) removals of more than 80 to 90%. With very dilute wastewaters, such as municipal sewage, this value may be closer to 50%. Conversely, with very concentrated wastes, the total BOD removal efficiency achieved may be much higher, but the residual BOD concentration could still exceed several thousand mg/L.
For wastewaters with BODs or biodegradable CODs below 2,000 mg/L, aerobic processes predominate. Between 1,000 and 30,000 mg/L, anaerobic wastewater treatment technology can be applied in either low- or high-rate forms. For very concentrated wastes containing more than 20,000 ‒ 30,000 mg COD/L, or for high concentrations of suspended solids, low-rate anaerobic digestion is usually chosen.
To give you an idea of the difference between low- and high-rate digestion the authors state the following...
Anaerobic digestion is well known as a treatment process for high-strength wastes such as sludges and manures that contain elevated levels of suspended solids. When the majority of the organic material is insoluble, lengthy digestion periods are required to allow for the relatively slow biological process of hydrolysis and solubilization of the insoluble materials. Once solubilized, the dissolved organics can undergo further conversion to volatile organic acids and methane fairly rapidly. To permit anaerobic digestion of particulates, total digester retention times of at least ten to twenty days are normal.
In contrast, high-rate anaerobic treatment technologies are intended for wastewaters in which the organic pollutants are soluble. Since hydrolysis of organics is not required with soluble wastewaters, much faster conversion rates to methane can be obtained. This is one factor that has permitted the operation of high-rate anaerobic processes at retention time of less than eight hours.
If the ratio of total COD/soluble COD is greater than 1.0 for a given wastewater, complete removal of the COD can only be achieved by removing both soluble and particulate organics during treatment. High-rate anerobic processes do not provide adequate hydraulic retention time for digestion of most particulates.
Source: Malina, Joseph F., Jr. and Frederick G. Pohland. Design of Anaerobic Processes for the Treatment of Industrial and Municipal Wastes. Boca Raton, Florida: CRC Press, 1992.

Note: The image above has been slightly modified from the original but the COD conversions are identical.
Anaerobic Digestion Pathway

Source of Graphic: Mohan, S Venkata. “Fermentative hydrogen production with simultaneous wastewater treatment: influence of pretreatment and system operating conditions.” Journal of Scientific & Industrial Research. Volume 67, November 2008, pp. 950-961.
Effect of Temperature on Microbial Growth
In process control, accurate temperature measurements are helpful in evaluating process performance because temperature is one of the most important factors affecting microbial growth. Generally stated, the rate of microbial growth doubles for every 10 degree C increase in temperature within the specific temperature range of the microbe.
Source: United States Environmental Protection Agency. "Process Control Manual for Aerobic Biological Wastewater Treatment Facilities." EPA-430/9-77-006. March 1977.
An important point to note in the statement above is within the specific temperature range of the microbe.
Activated sludge systems typically operate in the Mesophilic range (see table below), though some industrial systems push into the Thermophilic range. What this means is that beginning at a temperature of 68 degrees F (20 degrees C) for the Mesophilic range, an increase in temperature in the bioreactor to 86 degrees F (an increase from 20 to 30 degrees C) will result in a doubling of bacterial growth. But this does not mean that a reduction in temperature to 50 degrees F (decreasing from 20 to 10 degrees C) will cause a 50% reduction in bacterial growth because 50 degrees F (or 10 degrees C) is outside the range for which this oft-stated temperature rule-of-thumb applies.

It should also be recognized that as the temperature in the bioreactor increases above 95 degrees F (35 degrees C), the growth rate doubling effect per 10 degree C increase will not continue to hold due to the high-temperature stress bacteria are being subjected to, a statement I am making based on my experience. To be clear, the temperature classification table reproduced above states the "optimum" temperature range for Mesophilic bacteria to be 25 to 40 degrees C (77 to 104 degrees F), but I have seen repeatedly that as the temperature climbs above 35 degrees C (95 degrees F) in the bioreactor, plant operating conditions will begin to deteriorate as evidenced but an increase in small, dispersed solids being lost from the secondary clarifier.
For a more in-depth analysis of temperature please go to my blog post entitled "Wastewater Temperature."
Carbon Adsorption
Activated carbons, both powdered and granular, are made from a wide variety of carbonaceous starting materials: coals (anthracite, bituminous, lignite), wood, peat, coconut shells, etc. They are manufactured in such a way that they have a tremendous network of pores inside, and the total surface area inside such carbons is typically 500 to 1,500 square meters per gram, a huge amount. It is this extensive surface on which adsorption of organics can occur. Adsorption amounts up to as high as 0.30 g organic/g carbon are not unusual.
Source: Cooney, David O. "Adsorption Design for Wastewater Treatment." Boston: Lewis Publishers, 1999.
Heavy Metals Impact on Activated Sludge
The table shown below, which can be found in another location on this site, is from a 1977 EPA manual called "Process Control Manual for Aerobic Biological Wastewater Treatment Facilities." I have reproduced the information which shows the allowable concentrations of 13 metals in the influent to an activated sludge process. I know a data of 1977 might be considered to be too out-of-date but this is the only source for this type of information I've been able to come across.

Activated Sludge Table
Below is a handy table reproduced from Metcalf & Eddy's Wastewater Engineering Treatment and Reuse textbook. If you click on the image below the table will open as a full-size page in the form of a PDF file for easier viewing and printing.
Activated Sludge Nutrient Requirements
The biomass requires nitrogen and phosphorus in order to effect synthesis, metabolism, and removal of organics in the treatment process. The "rule of thumb" to assure adequate nitrogen and phosphorus for BOD removal is to provide a maximum nutrient mass ratio of 100:5:1 (BOD:N:P). A higher ratio (e.g., 150:5:1) will reduce the rate of BOD removal and promote filamentous growth.
Source: Eckenfelder, W. Wesley and Jack L. Musterman. "Activated Sludge Treatment of Industrial Wastewater." Lancaster, PA: Technomic Publishing Co., Inc. 1995. (pgs. 40 to 41)
As a side note, if you need to know the concentration of "polyphosphate" in the wastewater you determine this by measuring both the total phosphate and orthophosphate concentrations. The difference between these two values is the polyphosphate concentration. I use Hach's TNT chemistries for the majority of my lab work. Hach offers tests spanning a wide range of concentrations so you either need to know the concentration range you are working with or you will need to do "range-finding" where you have to do several iterations using different test kit ranges to find the actual range for your sample. For the test kit shown below the total phosphorus test requires one hour of digestion at 100 degrees C along with the orthophosphate (reactive phosphorus) test which takes just 10 minutes.


Primary Clarifier Performance
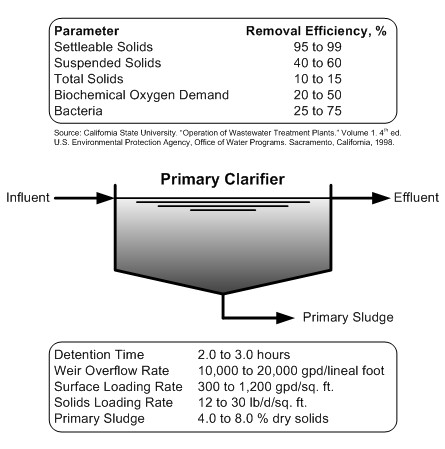
Wastewater Plant Unit Process Performance

Refinery API Separator Performance

